Cosmic ray spallation
| Nucleosynthesis |
|---|
 |
| Related topics |
Cosmic ray spallation, also known as the x-process, is a set of naturally occurring nuclear reactions causing nucleosynthesis; it refers to the formation of chemical elements from the impact of cosmic rays on an object. Cosmic rays are highly energetic charged particles from beyond Earth, ranging from protons, alpha particles, and nuclei of many heavier elements. About 1% of cosmic rays also consist of free electrons.
Cosmic rays cause spallation when a ray particle (e.g. a proton) impacts with matter, including other cosmic rays. The result of the collision is the expulsion of large numbers of nucleons (protons and neutrons) from the object hit. This process goes on not only in deep space, but in Earth's upper atmosphere and crustal surface (typically the upper ten meters) due to the ongoing impact of cosmic rays.
The process[edit]
Cosmic ray spallation is thought to be responsible for the abundance in the universe of some light elements—lithium, beryllium, and boron—as well as the isotope helium-3. This process (cosmogenic nucleosynthesis) was discovered somewhat by accident during the 1970s: models of Big Bang nucleosynthesis suggested that the amount of deuterium was too large to be consistent with the expansion rate of the universe and there was therefore great interest in processes that could generate deuterium after the Big Bang nucleosynthesis. Cosmic ray spallation was investigated as a possible process to generate deuterium. As it turned out, spallation could not generate much deuterium, but the new studies of spallation showed that this process could generate lithium, beryllium and boron; indeed, isotopes of these elements are over-represented in cosmic ray nuclei, as compared with solar atmospheres (whereas hydrogen and helium are present in about primordial ratios in cosmic rays).
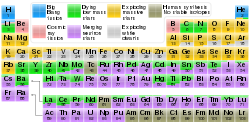
The x-process in cosmic rays is the primary means of nucleosynthesis for the five stable isotopes of lithium, beryllium, and boron.[1] As the proton-proton chain reaction cannot proceed beyond 4He due to the unbound nature of 5He and 5Li,[2] and the triple alpha process skips over all species between 4He and 12C, these elements are not produced in the main reactions of stellar nucleosynthesis. In addition, nuclei of these elements (e.g. 7Li) are relatively weakly bound, resulting in their rapid destruction in stars and no significant accumulation. It was thus postulated that another nucleosynthesis process occurring outside stars was necessary to explain their existence in the universe. This process is now known to occur in cosmic rays, where lower temperature and particle density favor reactions leading to the synthesis of lithium, beryllium, and boron.[1]
In addition to the above light elements, tritium and isotopes of aluminium, carbon (carbon-14), phosphorus (phosphorus-32), chlorine, iodine and neon are formed within solar system materials through cosmic ray spallation, and are termed cosmogenic nuclides. Since they remain trapped in the atmosphere or rock in which they formed, some can be very useful in the dating of materials by cosmogenic radionuclide dating, particularly in the geological field. In formation of a cosmogenic nuclide, a cosmic ray interacts with the nucleus of an in situ solar system atom, causing cosmic ray spallation. These isotopes are produced within earth materials such as rocks or soil, in Earth's atmosphere, and in extraterrestrial items such as meteorites. By measuring cosmogenic isotopes, scientists are able to gain insight into a range of geological and astronomical processes. There are both radioactive and stable cosmogenic isotopes. Some of the well-known naturally-occurring radioisotopes are tritium, carbon-14, and phosphorus-32.
The timing of their formation determines which nuclides formed by cosmic ray spallation are termed primordial and which are termed cosmogenic (a nuclide cannot belong to both classes). The stable nuclides of lithium, beryllium, and boron found on Earth are thought to have been produced by cosmic ray spallation predominantly before the solar system's formation (thus making these primordial nuclides, by definition) are not termed "cosmogenic," even though they were formed by the same process as the cosmogenic nuclides (although at an earlier time). In contrast, the radioactive nuclide beryllium-7 falls into this light element range, but this nuclide has a half-life too short for it to have been formed before the formation of the solar system, so that it cannot be a primordial nuclide. Since the cosmic ray spallation route is the most likely source of beryllium-7 in the environment, it is therefore cosmogenic.
See also[edit]
References[edit]
- ^ a b Greenwood & Earnshaw 1998, pp. 13–15.
- ^ Coc, A.; Olive, K. A.; Uzan, J.-P.; Vangioni, E. (2012). "Variation of fundamental constants and the role of A = 5 and A = 8 nuclei on primordial nucleosynthesis". Physical Review D. 86 (4): 043529. arXiv:1206.1139. doi:10.1103/PhysRevD.86.043529.
- Greenwood, N. N.; Earnshaw, A. (1998). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-7506-3365-9.
Further reading[edit]
- Meneguzzi, M.; Audouze, J.; Reeves, H. (1971). "The production of the elements Li, Be, B by galactic cosmic rays in space and its relation with stellar observations". Astronomy and Astrophysics. 15. Bibcode:1971A&A....15..337M.
External links[edit]
- Ray Isotope Spectrometer[permanent dead link]
- University of Leeds paper[permanent dead link], proceedings of the 26th ICRC.(Link down, Jan 2011)
- Heavy Cosmic Ray propagation Using New Spallation Cross-Section Expressions[permanent dead link], proceedings of the 26th ICRC.(Link down, Jan 2011)
- Evidence for cosmic ray spallation production of helium and neon found in volcanoes